What Clinicians Need to Know About the Pfizer-BioNTech COVID-19 Vaccine for the administration to allergic patients.
We would like to thank allergist Dr. Vasiliki Vourga for sharing the specific information and guidelines issued by the Centers for Disease Control and Prevention of America regarding the administration of the Pfizer-BioNTech COVID-19 vaccine to Allergic Patients. (Amanda Cohn, MD Sarah Mbaeyi, MD, MPH - Centers for Disease Control and Prevention CDC)
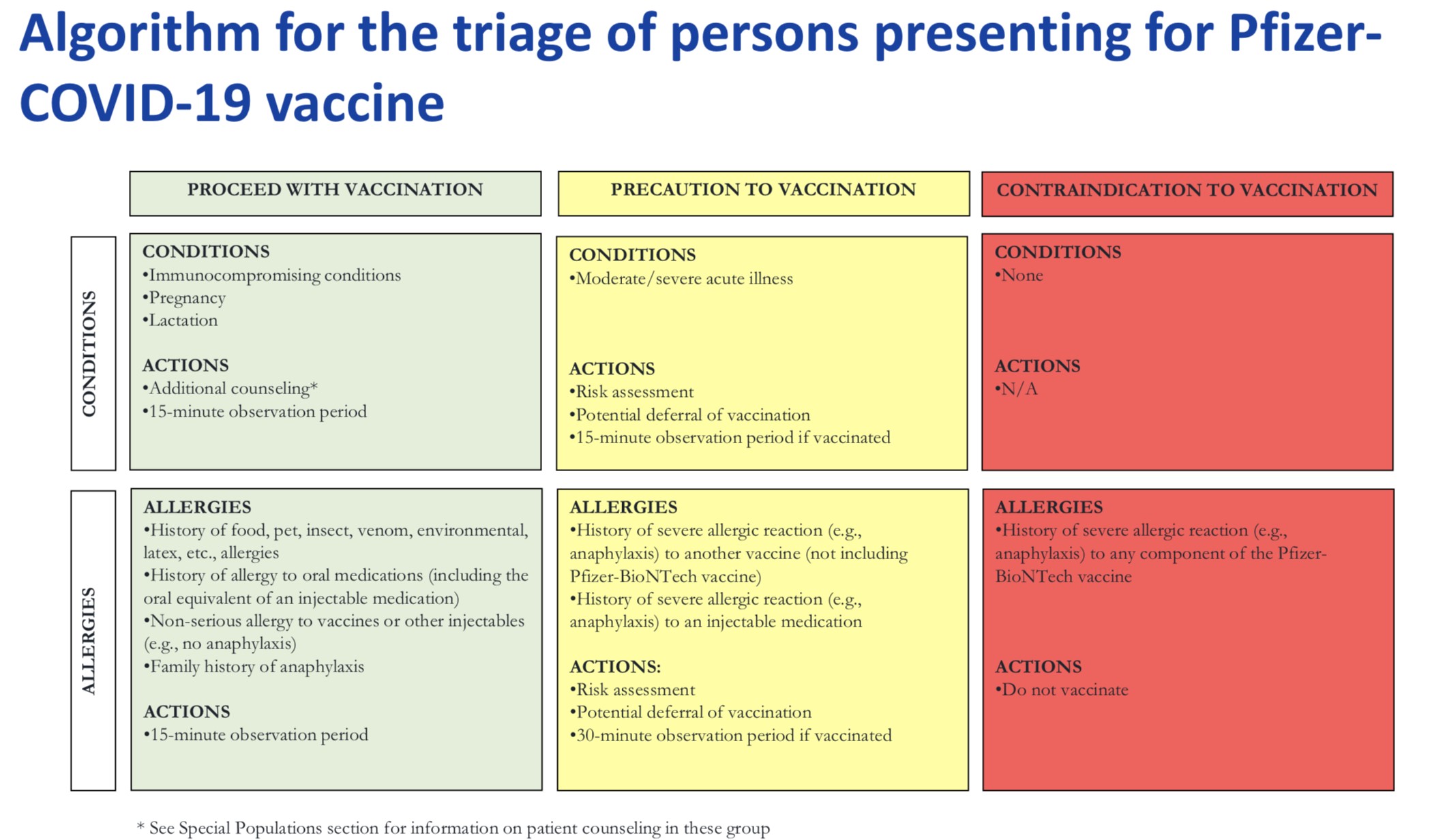
Contraindications and precautions
Package insert:
– Severe allergic reaction (e.g., anaphylaxis) to any component of the Pfizer-BioNTech COVID-19 vaccine is a contraindication to vaccination.
– Appropriate medical treatment used to manage immediate allergic reactions must be
immediately available in the event an acute anaphylactic reaction occurs following administration of the vaccine.
Because of reports of anaphylactic reactions in persons vaccinated outside of clinical trials, the additional following guidance is proposed:
– A severe allergic reaction to any vaccine or injectable therapy (intramuscular, intravenous, or subcutaneous) is a precaution to vaccination at this time.
– Vaccine providers should observe patients after vaccination to monitor for the occurrence of immediate adverse reactions:
• Persons with a history of anaphylaxis : 30minutes